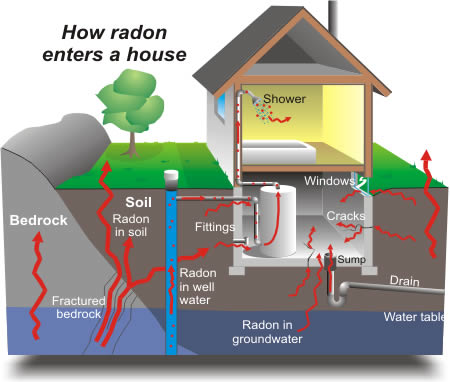
Radioactive radon is an inert (i.e., not chemically reactive), colorless, odorless gas, one in a chain of natural by-products of uranium decay. Radon is produced from the decay of radium released from uranium ore, which is ubiquitous in soils and rock worldwide. As radon forms in the earth, it rises to the surface where it dissipates rapidly in the air.
However, when radon enters residential and other tightly enclosed structures, its concentration can rise to levels that increase cancer risk, particularly when inhabitants of homes with higher radon levels are exposed over a period of years. Inhaled radioactive alpha particles produced by radon’s two short-lived decay products can directly or indirectly damage DNA in lung cells.
The following video is a visual representation of alpha radiation filmed in a laboratory. The controlled atmosphere creates the right conditions to see the vapor trails left by alpha particles as they are released from they’re radium molecules.
Radon is the second leading cause of lung cancer in the United States and the leading cause of lung cancer among people who have never smoked. People who smoke and are exposed to raised levels of radon have a much higher risk of lung cancer.
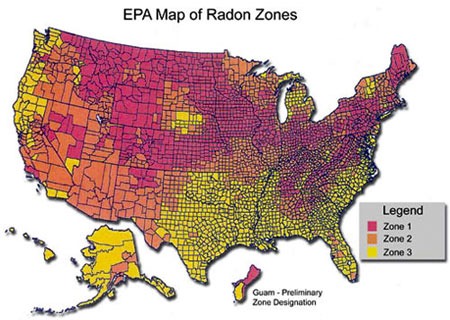
The U.S. Environmental Protection Agency (EPA) action level for residential radon (the level at which remedial action is recommended) is four picocuries per liter of air (4 pCi/L), based both on risk considerations and the technical feasibility of remediation. Up to 6 percent of U.S. homes are estimated to have radon concentrations at or above the action level. Yet most radon-induced lung cancers arise from exposures below that level.
In 2009, the World Health Organization (WHO) revised its recommendation for a maximum acceptable radon concentration in a residential dwelling to 2.7 pCi/L.